 domoyega
domoyegaEU Mercury Regulation: Focus on dental amalgam
In February 2016, the European Commission proposed a regulation on mercury as part of implementing the United Nation’s International Minamata Convention on Mercury.
MS – 12/2016
In order to protect health and the environment, the regulation sets high standards for, inter alia, the use of dental amalgam.
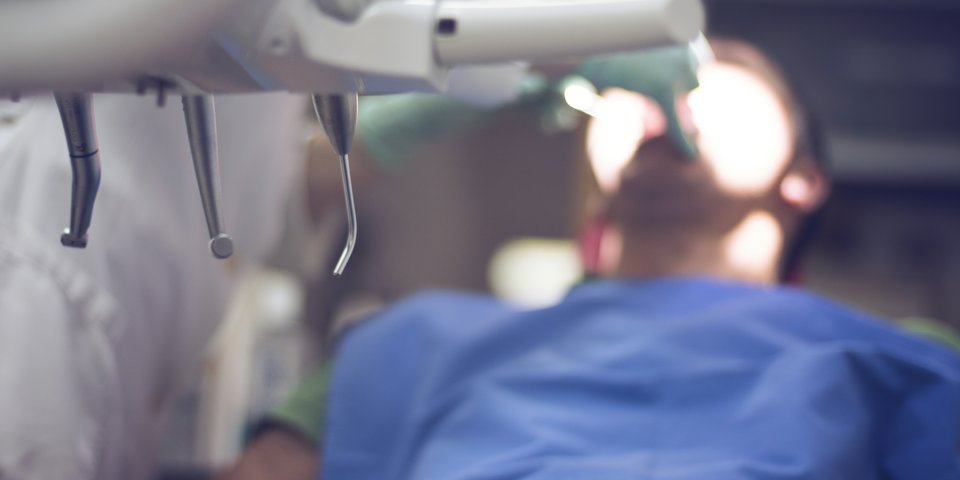
In October 2016, the European Parliament’s Health Committee (ENVI) voted to phase out mercury in dental amalgam by the end of 2022. The GKV-Spitzenverband welcomes the goal of protecting health and the environment by strengthening regulations concerning the use of dental amalgam. The proposal to only use dental amalgam in encapsulated form in the future makes sense and is already the practice in Germany. The proposal for dental facilities across Europe to be equipped with amalgam separators to retain and collect amalgam residues is also an appropriate means of preventing mercury entering the environment.
However, a ban on the use of dental amalgam is not necessary. In terms of dental care, amalgam should continue to be available as a filling material. It has proven itself over time to be a filling material with positive properties. It is superior to other filling materials in terms of its workability and longevity. The study of alternative material such as composites is far less advanced. However, there are also reports here of intolerances or toxic risks.
Therefore, the GKV-Spitzenverband views amalgam as a cost-effective option for filling material in dental treatment. Even in the future, it should be available as one of the options for filling material in the interest of patients and contribution payers.
Discussions between the European Parliament, the EU Council and the European Commission over the EU Mercury Regulation were concluded at the start of December 2016. As a compromise, there will be no ban on amalgam in the EU, as was called for by the European Parliament. Rather, the EU Member States gathered in the Council have demanded that amalgam continue to be used as a filling material for the time being due to security of supply. However, the European Commission will also submit a feasibility study by 30 June 2020 for the phase-out, which should possibly be completed by 2030. This should take into consideration the competence of the Member States for designing and structuring their health systems.
The Member States have until 1 July 2019 to develop national strategies for the phase-out by 2030. In addition, the use of dental amalgam in vulnerable groups (pregnant women, women who have recently given birth, children under the age of 15) will be banned as of 1 July 2018.
