 thecorgi
thecorgi
Collecting and exchanging health data – Opportunities for Europe
“Data is the new gold”. This saying is increasingly associated with the digital age when it comes to collecting and using data.The main features of the EHDS
ed* Nr. 02/2022 – Chapter 2
The main features of the EHDS
“Data is the new gold”. This saying is increasingly associated with the digital age when it comes to collecting and using data. The European Commission already realised a long time ago that enormous added value can be derived from data. According to its estimates, The volume of data worldwide will increase by 530 per cent in 2025 compared to 2018.1 And it wants to tap into this continuously growing treasure trove of data. How it would like to implement this, the European Commission explained in its European Data Strategy in February 2020. The aim is to create a single European and cross-border market for data, based on European rules and values. In the course of this, nine sector-specific data spaces are to be created, among other things, in the areas of health, mobility, energy, finance or industry.
Primary and secondary data use
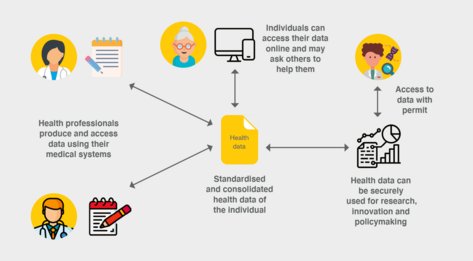
It starts with the healthcare sector and the idea of building an EHDS that regulates exactly when, how and for what data can be collected and exchanged. Citizens should be given access to their data. The aim is to improve healthcare in the EU.
On 3 May 2022, the European Commission presented its idea for an EHDS. The aim is to bring together health data in a meaningful way and to use it across borders for research, innovation, policy-making and healthcare.

In order to make this possible, some challenges have to be overcome. Thus, the EHDS must it well into the already existing national health and social structures. These differ greatly in the Member States, for example in the level of digitisation, the technical governance structures and the data protection conventions. While in Germany, for example, the ePrescription (eRezept) will only be introduced gradually from September, it has already been used in Finland for 15 years. European harmonisation of these national structures and standards is costly.
Two regulatory areas under one roof
With its draft regulation, the European Commission places two different regulatory areas under one legislative initiative and distinguishes between primary and secondary data use.
Access to treatment data
In a first step, patients should have the right to access their treatment data electronically anywhere in the EU and make it available to their attending physicians. This can have great advantages for patients. For example, a doctor in the Czech Republic treating a Portuguese patient should be able to access his or her basic health information. A German patient posted in Helsinki should also be able to obtain his or her prescription medication from a Finnish pharmacy. Prescriptions should be digitally retrievable and redeemable throughout Europe. This harmonisation should not only reduce the costs of health data traffic in the EU, but also strengthen cross-border mobility in Europe and, above all, improve the quality of healthcare for citizens.
For this purpose, patients are to be given access to their electronic health data. Service providers, such as doctors or hospitals, must be connected to an interoperable data infrastructure, be able to read data in their national language and add data records. When filling ePrescriptions, patients must be able to identify themselves and pharmacy staff must be able to read prescriptions issued abroad.The interoperable data structure already exists, in principle in the MyHealth@EU project. It has also been tested by some Member States, so the EHDS can build on this structure. The voluntary exchange of data via MyHealth@EU is to become mandatory for all Member States with the EHDS.

